Watson Health
Introducing a new product into the clinical development space
As part of a 2-person design team, I introduced a net new product to the clinical development space. Supported by clinical trial data, we created a product which aimed to drastically cut down on the time and resources lost when developing a clinical trial protocol. IBM Study Advance helped authors build quality protocols by providing both the power of collaboration and data insights to help eliminate guesswork and enable efficiency and informed decisions across the entire protocol development process.
I worked closely with one other hybrid designer, a product manager, and a development team to launch this product from the ground up, encompassing the entire product lifecycle. I operated as design strategist, user researcher, ux designer, and visual designer.
Overview
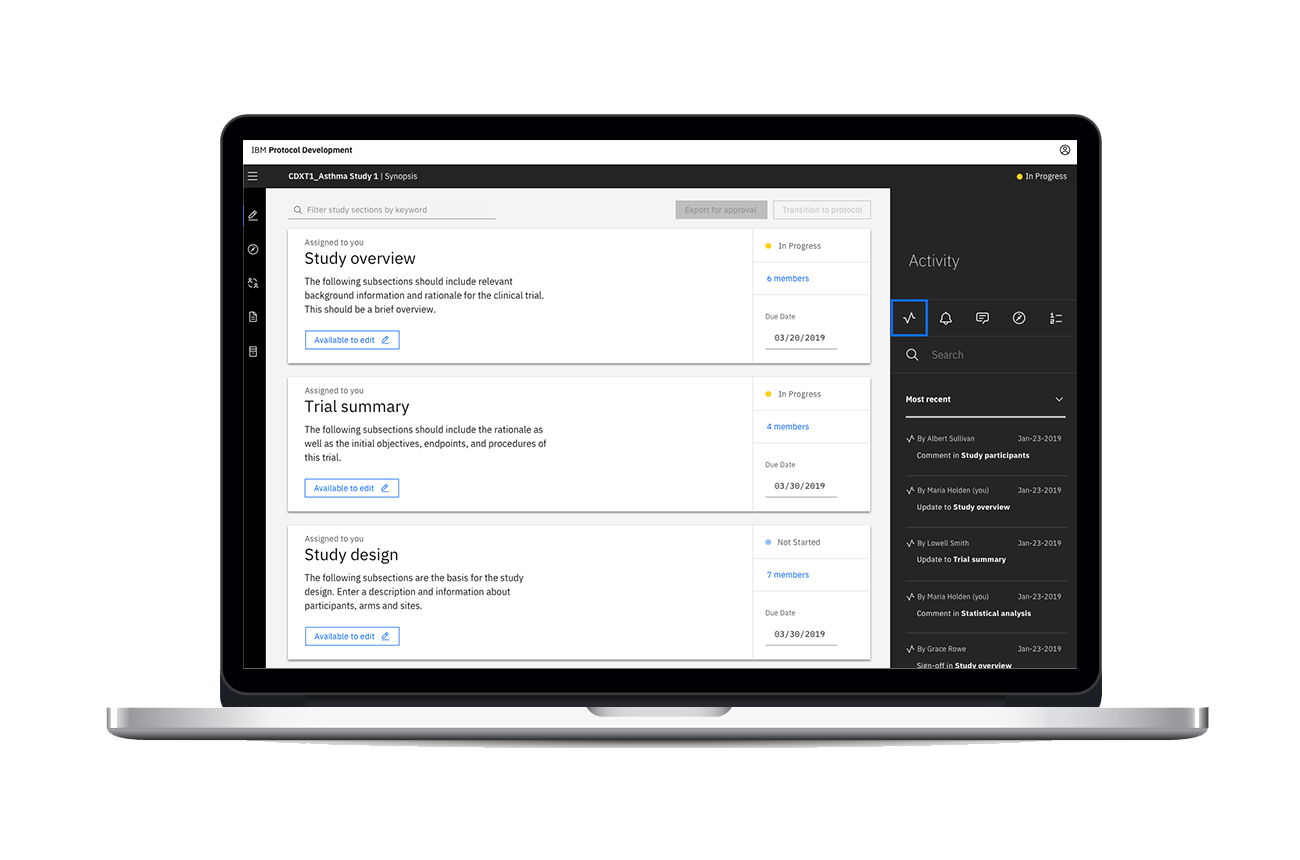
Study detail page with expanded Activity Feed
Developing a clinical trial is a critical milestone in the complex process of bringing safe, viable, and effective treatments to patients. Often the most time consuming and costly challenges facing a clinical development team are disjointed collaboration and the protocol amendments that arise from patient recruitment difficulties. According to R&D and clinical trial management expert Kenneth Getz, these amendments add an average of 500,000 USD of unplanned costs and an additional 61 days to the development timeline. Clearly, there was opportunity to save time and money by introducing sound UX design principles and valuable insights at key moments.
The Challenge
“Almost 80% of all clinical trials
fail to finish on time.”
Slide from Research Synthesis playback depicting clinical trial authoring journey; pain points shown by severity
To kick off the project, stakeholders gathered in Durham, North Carolina to workshop the problem space and to establish the guiding principles to be used to attack it. We learned the following about clinical development teams:
• Various subject matter experts contribute to the writing of the protocol at multiple stages
• Teams are spread across timezones and work asynchronously
• A patient profile is created to determine the trial participants
• A master document is passed between team members via email
This helped to consolidate and prioritise key pain points and opportunities for improvement:
• Collaboration and version history
• Patient population insights— protocol amendments consistency result from creating a patient profile that is difficult to recruit
Immediately we focused on improving the ability for team members to work together at different times and in different places while incorporating clinical trial data to improve the decisions made about patient profiles and eliminate costly protocol amendments.
Less than 8 months from kickoff, we launched a robust product that facilitated collaboration and provided insights to inform study design decisions. In between there were daily stand-ups, weekly playbacks, dozens of user interviews and user tests (+ hours and hours of interviews synthesised), and hundreds of wireframes created.
The end result was a product that vastly improved the day to day work of its users and increased the viability and effectiveness of life-changing treatments.
The Approach
Product information architecture map
Slides from Research Synthesis playback
Interestingly, this was both the most rewarding and least successful product I have contributed to. Simply put, the company did invest in the data that would move this product from a pleasant user experience to industry-shifting. The feedback in user testing sessions was consistent— the execution of the design was excellent but the data was lacking. The pool from which the data was drawn was limited to US-based trials in the last 30 years. Potential clients loved the idea of being able to collaborate on a study in real-time in a centralised, digital, and secure location but they could not invest in licensing the product because their trials were run all across the world. On the other side, the company could not/would not invest in acquiring more patient data from across the world when there was not a dedicated and paying user base. This was a classic corporate catch-22.
The learning was that business outcomes and user outcomes have to follow the same trajectory in order for a product to succeed. In an ideal world a leap of faith could have been taken by either the company to invest in more data or a client to invest in a license with the hopes that the data would eventually improve. There are exceptions, but Fortune 500 companies typically act on safe bets rather than leaps of faith. Though the balance can be hard to strike, the role of a designer is to both emphasise the needs of the end user AND target business outcomes so that safe bets are readily taken on the products we create.
Outcomes
Project dashboard with expanded Notifications panel
Section detail showing in-progress cohort creation
Product guidance modal